
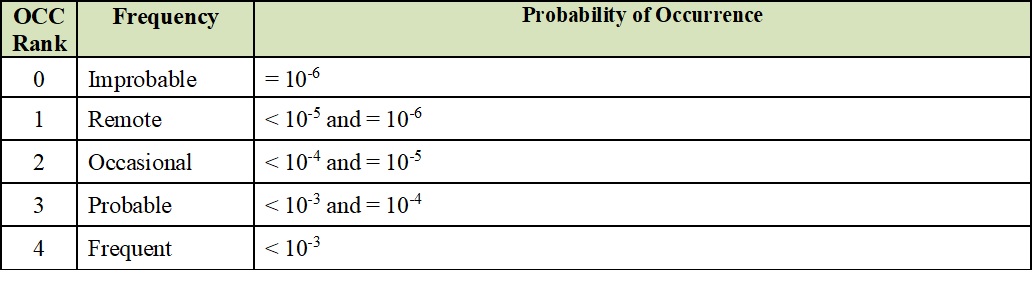
Authorization and oversee the creation of Quality Risk Management procedures that include the requirements established in this SOP.Quality head/designee shall be responsible for.To facilitate continuous improvement in manufacturing resulting from knowledge gained through periodic Quality Risk Management system reviews.Īlso read: SOP for Audit Trail Review and Privilege Policy.To assure coordination of quality risk management across the various functions and departments.The level of effort, formality, and documentation of the quality risk management process should be aligned with the level of risk.The evaluation of the risk to quality is based on scientific knowledge and ultimately linked to the protection of the patient.Manufacturing head/designee shall be responsible to ensure the following principles of Quality Risk Management are applied:.
Providing leadership for the risk management process to ensure that ongoing Quality Risk Management processes operate effectively.Manufacturing head/designee shall be responsible to demonstrate a commitment to the risk management process by:.This SOP is applicable to the management of all types of risk events that have a potential threat to product quality, facility, organization, etc.


 0 kommentar(er)
0 kommentar(er)
